03
Sep
2024
The NMC seeks to understand views on remote prescribing practices.
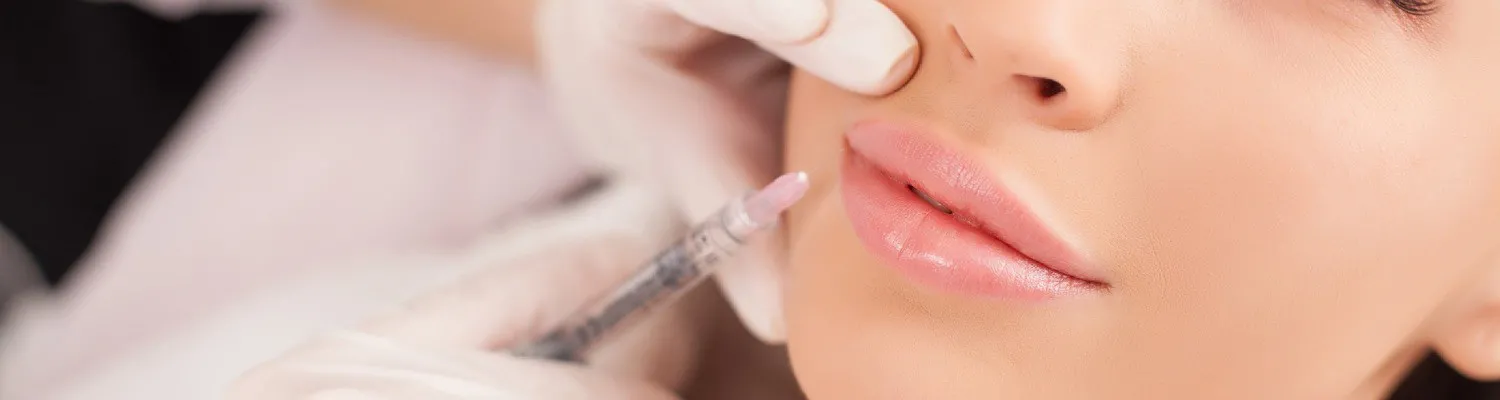
The current position on the remote prescribing of injectable cosmetic products is under review. This includes certain anti-wrinkle and weight loss injections, as well as aesthetic emergency kit items.
The goal is to strengthen guidance as part of the regulatory role and commitment to protecting the public. This effort also aims to better align with other healthcare regulators who require professionals to conduct face-to-face consultations before prescribing non-surgical cosmetic medicines.
Why is input being sought?
Many professionals in the cosmetic field provide safe and effective care daily. However, there is inconsistent regulation around non-surgical cosmetic practices.
There are specific concerns regarding the remote prescribing of prescription-only medicines for non-surgical cosmetic procedures. Unlike other areas of prescribing, this can occur without adequate access to medical records or direct contact between the prescriber and the patient. This limits the prescriber’s ability to assess individuals holistically when determining whether it is safe and appropriate to prescribe certain medicines.
Who needs to provide input?
Input is being sought from members of the public, professionals, employers, and stakeholders across the UK.
The NMC is particularly interested in hearing from:
Gathering these views will help to understand the impact of any potential changes on those administering and receiving non-surgical cosmetic medicines. It will also ensure that any changes made are in the public's best interest and support professionals in delivering the high standard of care that people have the right to receive.
It is important for people and communities to help shape this work, and a roundtable event and focus groups will be hosted starting in October to gather views. Those interested in participating can express their interest by emailing educationandstandards@nmc-uk.org.
Updates will also be provided on the NMC's website and social media channels in the coming weeks, and subscribers to newsletters are encouraged to stay informed.
Anne Trotter, NMC Assistant Director of Education and Standards, said:
“Nurses and midwives are integral to the safe and effective delivery of person-centred care, including the prescribing and administration of medicines. They are entrusted to provide high-quality care that is evidence-based and in line with our Code and standards – but we know concerns remain about the remote prescribing of non-surgical cosmetic medicines.
“We’re clear that remote prescribing is unlikely to be suitable for injectable cosmetics. However, we want to strengthen our position on what safe and effective prescribing practices look like, so that we can deliver on our primary purpose of protecting the public.
“We look forward to hearing from the public, employers, professionals and our stakeholders, and we’ll provide more information over the coming weeks about how people can engage with us.”