
24
Feb
2025
Authors:
Dr. Nadav Pam (M.D) Formatk Systems, Israel
Lacey Allen Senior Hair Removal Technician, Canada
Amanda Killan Senior Hair Removal Technician, Canada
Shannon Roche Senior Hair Removal Technician, Canada
Not all diode lasers are equal. This whitepaper presents clinical proof of how POWER-MOTION™ technology enhances treatment speed, efficiency, patient comfort, and practitioner ease - solving key challenges in laser hair removal. Backed by real-world results, it’s a must-read for those staying ahead with the latest advancements.
Background:
The global laser hair removal market is projected to experience significant growth in the coming years. According to a recent global market forecast report, the market size was valued at USD 1,129 million in 2024 and is expected to reach USD 5,362.06 million by 2032, growing at a compound annual growth rate (CAGR) of 18.9% during the forecast period (1).
Similarly, Fortune Business Insights reports that the market was valued at USD 1.05 billion in 2023 and is projected to grow to USD 4.61 billion by 2032, exhibiting a CAGR of 18.1% (2).
Based on these projections, the estimated global market value for laser hair removal in 2025 would be approximately USD 1.6 billion to USD 1.7 billion. This growth is attributed to factors such as increasing demand for non-invasive cosmetic procedures, advancements in laser technology, and growing awareness of the benefits of laser hair removal.
Until today, the hair removal treatment modes considered the most popular and influential were the first-generation "Single" mode (also known as “stamping”) and the second-generation "Fast" Mode (also known as "In-motion" mode).
However, FormaTK Systems Ltd recently introduced a new hair removal treatment mode concept titled 3rd generation POWER-MOTION as part of the ALPHA and MAGMA Spark Pro Systems with the non-invasive Diode lasers with 808nm wavelengths.
There are two critical anatomical targets for the inactivation of hair follicles:
Laser hair removal technology is based on the absorption of energy by the melanin (endogenous chromophore) in the hair follicle in the anagen phase and the diffusion of the power into the dermal papillae and surrounding stem cells.
Laser hair removal is achieved through follicular unit destruction in anagen phase based on the extended selective photothermolysis concept of heat diffusion (3-13).
Hence, by increasing the heat diffusion rate, as seen in the thermal images of the LLD laser Diode
808nm, the new 3rd generation POWER-MOTIONTM hair removal mode, we expect to see improved end-clinical treatment.
Aim of the Case Study:
This study aimed to assess the preliminary safety and clinical efficacy of hair removal using the third-generation POWER-MOTIONTM LLD 808nm non-invasive diode laser applicator, developed by FormaTK Systems Ltd., after a minimum of five treatment sessions.
Methods:
This single-center, prospective case study was conducted at Divine & Free, a luxury wellness
boutique and medical spa known for its advanced technologies, located in the Perron District of St. Albert, Canada. The study was carried out by a team of three senior hair removal technicians from Divine & Free: Lacey Allen, Amanda Killan, and Shannon Roche.
Six healthy patients (three females and three males), aged 35 to 42, participated in the study. Twelve anatomical areas were treated for unwanted body hair using the Alpha system, equipped with the LLD (large laser diode) 808nm wavelength diode laser, featuring a tip surface area of 15mm over 30mm (4.5cm2) in POWER-MOTIONTM mode.
Participants were selected based on the Fitzpatrick skin scale and assessed with the "Milo" melanin meter to ensure inclusion of healthy adults with skin types I to III. Each participant underwent at least five treatment sessions, spaced 4 to 8 weeks apart, with a follow-up evaluation conducted one month after the final treatment. Treatment parameters were individualized, with the LLD diode laser in POWER-MOTIONTM mode, utilizing energy settings between 20J/cm2 and 24J/cm2, and a fixed pulse duration of 40ms.
Written informed consent was obtained from all participants before the initiation of treatments.
Clinical photographs were taken by the technician team before and after the treatments, and these were evaluated by Dr. Nadav Pam, Clinical Director at FormaTK Systems Ltd.
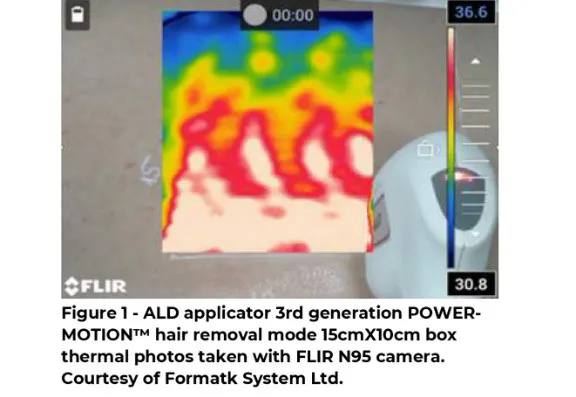
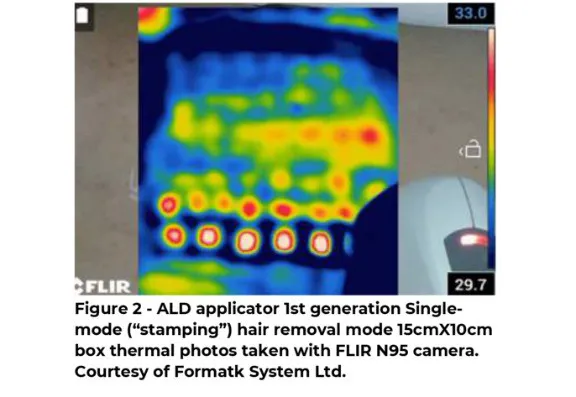
Inclusion criteria:
1. Healthy adult subjects aged between 18 and 70 who have unwanted body hair
2. Fitzpatrick skin types 1-3
3. All participants agree to refrain from exposure to the sun or solarium (solar lamps) during the whole study period
4. All patients will be informed about the study objectives, terms of treatments, eventual benefits, and adverse effects and will express their willingness to participate deliberately in this clinical study
5. All participants signed an appropriate informed
consent form.
Exclusion criteria:
1. Fitzpatrick skin type 4-6
2. Drug-induced photosensitivity (e.g., Isotretinoin, Retin A
3. Pregnancy and breastfeedin
4. Cancer
5. Epilepsy
6. Severe diseases
7. Auto-immune diseases
8. Frequent episodes of labial Herpes Simplex in case of face Treatment
9.Immunosuppressive pharmacologic therapy
10. Any other medical condition considered contraindicated to the treatment by the investigator.
11. Any other hair removal treatments such as drugs, topical creams/lotions, or other phototherapy medical devices.
Results:
A total of six healthy patients, comprising three females and three males, were successfully treated for unwanted hair across twelve anatomical zones.
The patients, aged between 35 and 42 years (average age 40), had Fitzpatrick skin types I to III.
Each patient underwent a minimum of five treatment sessions per anatomical zone.
No side effects were observed during the course of this case study.
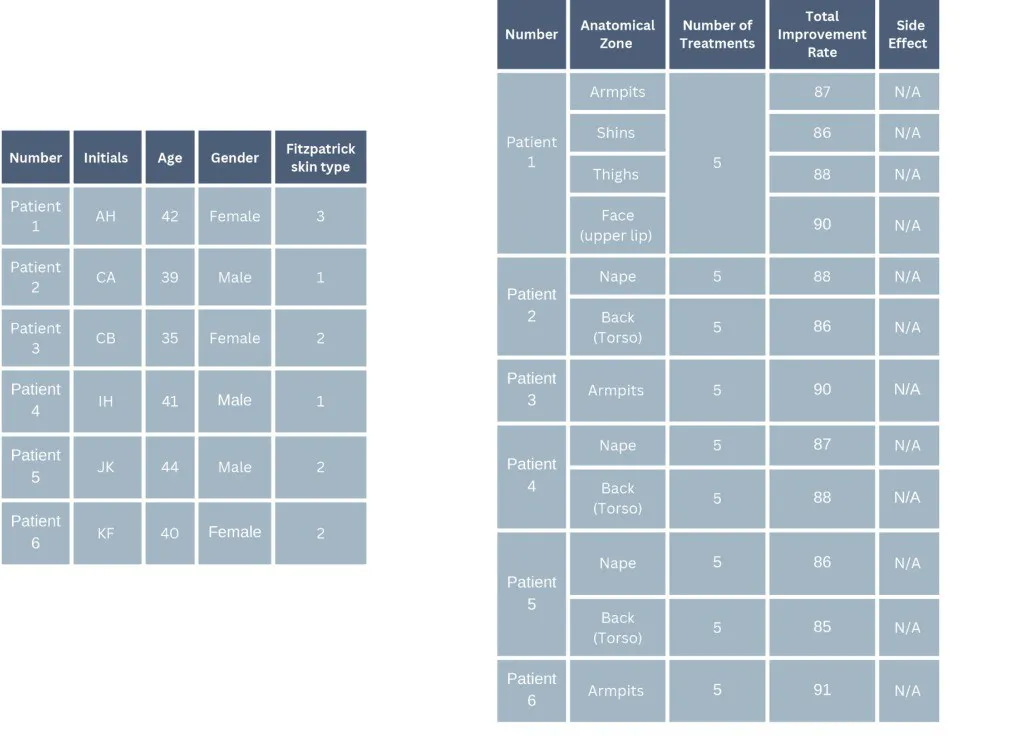
In conclusion:
This case study highlights the Alpha system with the third-generation POWER-MOTIONTM LLD Diode laser 808nm applicator as a safe and effective hair removal solution for patients with Fitzpatrick skin types I to III.
Discussion:
Enhanced Aesthetic Outcomes: All six patients experienced significant improvements across twelve anatomical treatment zones, as assessed using a 4-point evaluation scale.
Reduced Treatment Sessions: The third-generation POWER-MOTIONTM mode delivered significant improvement in all patients in just five treatment sessions.
Minimal Side Effects: Patients treated with the third-generation POWER-MOTIONTM mode reported no significant adverse effects. The only observed side
effects included transient pain and localized perifollicular erythema, both resolving within an hour post-treatment.
References:
Source: https://www.skyquestt.com/report/laser-hair-removal-market.
Source: https://www.fortunebusinessinsights.com/laser-hair-removal-market-103477
