Viveve is manufactured by an American women’s sexual health company based in California and was introduced to the UK in 2015.
It’s targeted at treating vaginal laxity (especially post childbirth), which can cause a decrease in sexual satisfaction and physical sensations during intercourse, through the use of patented radiofrequency (RF) energy to produce vaginal tightening.
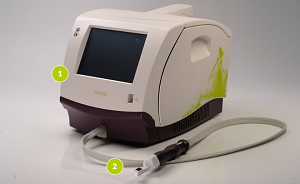
Viveve uses radiofrequency (RF) energy to selectively heat up the vaginal tissue and produce contraction. This causes tissue tightening and new collagen formation in the area.
The device itself consists of a table-top unit with a specially designed hand-piece featuring a single-use treatment tip.

During a 30-minute procedure the tip is inserted just inside the vaginal opening (approximately a thumb’s depth inside) and the RF energy is delivered, in conjunction with cooling, as the tip is moved in a clockwise or anticlockwise rotation around the circumferential vaginal opening.
The pulsed delivery of RF causes collagen contraction in the underlying tissues, leading to the stimulation of new collagen formation over the following 30 to 90 days and continued improvement at 6 and 12 months.
Approved for use in 21 countries around the world including Canada, Europe (via European CE Mark) and various countries in Asia.
FDA approval for use in America is pending as of 2016.
Trained medical professionals only, ideally with gynaecological knowledge and experience.
Vaginal laxity following childbirth.
Results are expected to last up to at least a year based on current studies. There is currently no clinical data available to show long term results after one year.
During a Viveve treatment, the overlying tissue is not damaged so there is said to be little to no downtime or pain and no local anaesthesia is required during the procedure.
Typical complications noted include redness, swelling, slight abdominal discomfort, white vaginal discharge, tingling or altered sensation after treatment – all are said to be mild and temporary, resolving quickly.
Rarely infections can occur. The manufacturers do also note that although it has never been reported and did not happen in trials, there is a potential risk of damage to the urethra or rectum.
As long as you are generally healthy and don’t have any skin diseases or infections in the area treated, there are few medical reasons why you should not undergo this treatment.
It is advised not to have the procedure done whilst you have having your monthly period (menstruation) as the increase in blood in the area can impede the targeting and penetration of the radiofrequency into the tissue within the vaginal canal, making treatment less effective.
If you are concerned that you have a sexually transmitted disease (STD) then this should be checked, diagnosed and treated, if required, before having a Viveve treatment.
You may well be asked to provide results from a PAP smear test that you have had within the last 12 months, or have a smear test before treatment, if it has been several years since you were last tested. The results of this will be used to evaluate suitability for treatment in case of any abnormal results.
Pregnant women are advised to wait to until after the birth of their child before having treatment.
A treatment can be completed in approximately 30 mins. Depending on the severity of the vaginal laxity, more than one session may be required.
The cost of Viveve treatment depends on the practitioner treating and the number of treatment sessions required.
To date the treatment has been tested in two prospective clinical trials in the USA and Japan which assessed the safety and efficacy of the treatment. The results from both studies showed that at 12 months after treatment vaginal tightness improved significantly (for 88% of women in the study), often to pre-childbirth levels, with a 68% mean increase in vaginal laxity scores, with no serious adverse effects reported in either study. No data after 12 months has been gathered, so the long-term effects and their longevity are currently unknown.