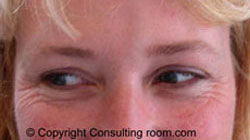
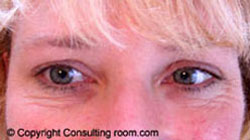
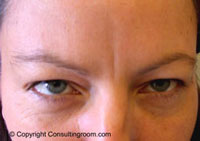
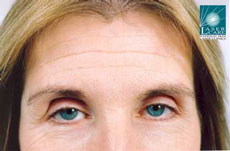
It may surprise you to know that botulinum toxin, or Botox® as we commonly now call it, has a history stretching back almost two hundred years. In 1822, a German doctor named Justinius Kerner, suggested that botulinum toxin injections might be used in the treatment of excessive sweating or hyperhidrosis. Botox® is now regularly used for this condition.
By the late 1960s, Botox® had begun to be used in American clinics for the treatment of squints (strabismus).
Its use in treatment of the eyes was pioneered by an American called Alan Scott. Twenty years later, the real breakthrough came in the wider application of Botox® when Allergan, the American manufacturer of Botox®, was granted approval to market the drug by the Federal Drugs Agency. This gave Allergan the license to sell its new product throughout the U.S. Since then, Botox® has been registered and used in over 90 countries around the world, and other versions of botulinum toxin are also available in the UK, namely type A variants Azzalure®, Bocouture®, Dysport®, Vistabel® and Xeomin® plus type B variant Neurobloc®.
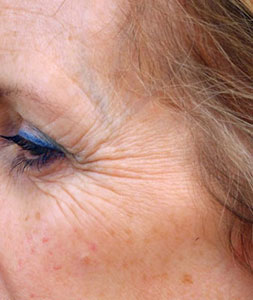
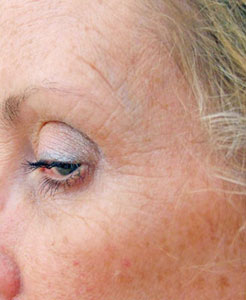
The next significant development in knowledge about botulinum toxin came in 1987 via a Canadian Ophthalmologist, Dr. Jean Carruthers. She had been treating her patients who suffered from blepharospasm with Botox®. Dr. Carruthers observed that a "side-effect" of this treatment was the reduction of crow's feet and wrinkles around the eyes. She then decided to test these findings scientifically, and she and her husband, a Consultant Dermatologist, (skin specialist) devised clinical trials to monitor the effectiveness of this treatment.
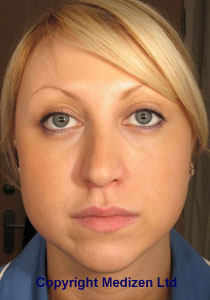
Since then, an enormous amount of clinical trial work and patient experience has been established using Botox® for the treatment of wrinkles, meaning that The American Society for Aesthetic Plastic Surgery (ASAPS) has consistently rated treatment with botulinum toxin as the most popular aesthetic procedure in the U.S.A. for many years.
Nearly a staggering 1,712,994 Americans had a Botulinum Toxin (Botox®, Dysport® or Xeomin®) treatment in 2019, a decrease of 4.9% on 2018 figures, but an increase of 17.8% when compared to 2015. This represents 55% of all non-surgical cosmetic treatments in the U.S.A. in that year.
Although there are as yet no similar detailed statistics available for the United Kingdom, it is likely that Botox® is also the number one procedure here.
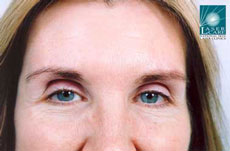
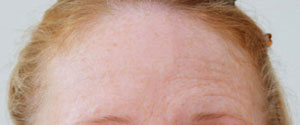
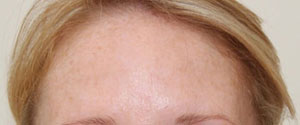
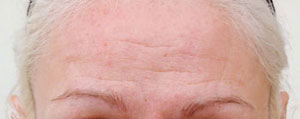
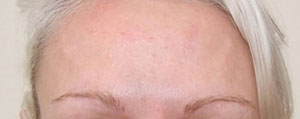
Botox® is now licensed by the regulatory authorities in the U.S., as well as more than a dozen other countries around the world, for the treatment of glabellar lines and wrinkles.
In March 2006, Vistabel®, the brand name used for Botox® with a dosing specific to treat glabellar lines was granted a licence in the UK from the Medicines and Healthcare products Regulatory Agency (MHRA) for the "temporary improvement in the appearance of moderate to severe glabellar lines (vertical 'frown' lines between the eyebrows) in adult women and men aged 65 and younger, when the severity of these lines has a psychological impact for the patient".
The same licence was granted to Dysport®, under the brand name Azzalure® in March 2009 and to Xeomin® under the brand name Bocouture® in July 2010.
However, if you are considering having a botulinum toxin treatment in this country, the above is nothing to be alarmed about. U.K. practitioners can use any of the brands available, however they should make you aware that they are using them outside of their current U.K. licensed indications if applicable.
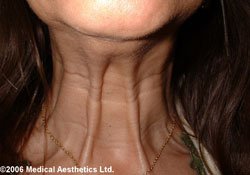
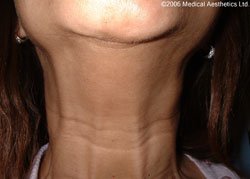
Science has discovered many other clinical uses for botulinum toxin including spasticity in adults and children, back pain, tension headache, migraine, dystonia, (spasm of the limbs) and anal fissure (tearing in the skin of the anus) which have all been treated successfully with this important product.
If you are considering treatment with botulinum toxin, the following information will give you a basic understanding of the procedure. It can't answer all your questions, since a lot depends on the individual patient.
Please ask a practitioner about anything you don't understand.