Comin' In HotRead our latest posts
There are many ways that menopause can affect the skin. Let's talk to an expert...
Did you know not all facial filler is the same? Each facial area needs and deserves tailored attention, specific products, and bespoke techniques to achieve the best and safest result!
Chair yoga offers a gentle introduction to helping build strength, flexibility, and confidence before moving on to more demanding forms of exercise.
patients often wait years before they gather the courage to book a consultation with an aesthetic doctor about potential tweakments.
From facials, to peels, to tweakments - we ask the experts how to get the bro glow…
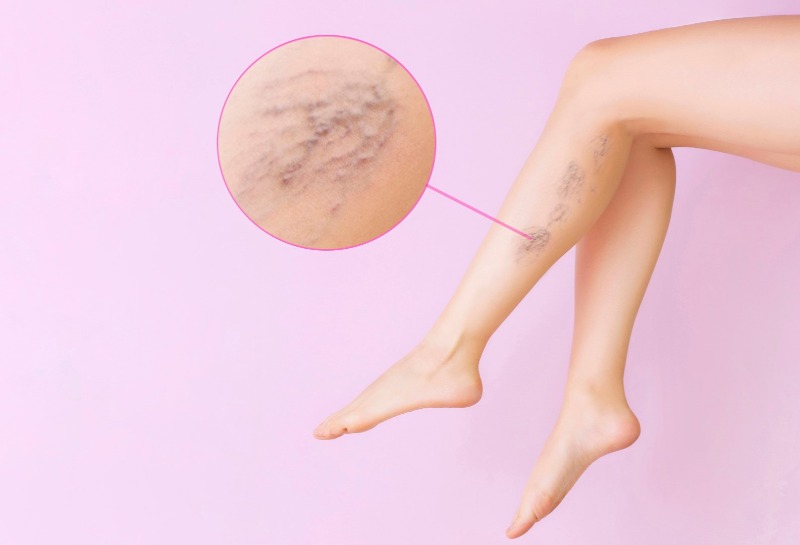
This is everything you need to know about choosing the right Microsclerotherapy Sclerosant in 2024...