30
Jun
2010
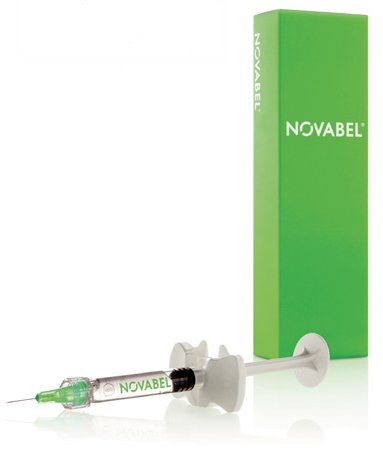 |
Merz Aesthetics has suspended sales of its new flagship facial shaping product, Novabel®, amid concerns following reports of a few patients treated with the product in the infra-orbital region who suffered adverse reactions.
In a letter out to its customers, Merz said; “Given the unique composition of Novabel an effective treatment has not yet been identified to quickly resolve these events. As practitioner and client satisfaction is a critical component of any facial aesthetic product Merz will temporarily suspend all sales and promotional activities for Novabel pending the availability of effective treatment protocols appropriate for highly sensitive areas.”
Launched at the IMCAS Paris meeting in January 2010 and available in 7 EU countries, to date an estimated 12,000 patients across Europe have been treated with Novabel.
It is uniquely based on alginate, a natural and biocompatible polysaccharide extracted from marine algae (or brown seaweed). The extracted alginate undergoes an intensive purification and stabilisation process to become soft, smooth, microscopic, three-dimensional gel spheres called Geleons™.
The Geleons have what is referred to as a ‘shape memory’ which means that they can pass through a thin needle and narrow tissue structures upon injection and are then able to recapture their original spherical shape once free of the restrictive space.
This flexibility enables the practitioner to literally ‘shape’ the treated area of skin after the injection of Novabel as the product remains supple enough for manual manipulation and massage to create the desired effect for approximately 30 minutes post injection. After this time the solution the Geleons are suspended in is absorbed into the skin’s tissues and they will then remain in position.
The product was marketed to treat multiple areas of the face such as for contouring the cheeks, chin and jawline and reducing the appearance of nasolabial (nose-to-mouth) lines, as well as treating sensitive areas such as hollows under the eyes or ‘tear troughs’.
Prior to launch, Merz Aesthetics conducted two prospective clinical studies involving 188 patients with up to18 months of safety and effectiveness data. Post-approval, (but 6 months prior to launch), additional explorative multicentre evaluations were performed (>250 patients) and a four-month multicentre experience campaign (>1900 patient treatments) was conducted.
“As of June 21st 2010, Merz Global Drug Safety has received 70 case reports from post-marketing surveillance. This number includes short-term transient adverse events such as redness, bruising, pain and swelling common with all filler/shaper products.
Included in these reports are 26 patients presenting with nodules and 10 patients with indurations, mostly in the infra-orbital area. Many of these have been resolved since being reported. Also included in these reports are 3 cases of confirmed granuloma.”
Merz has advised those practitioners certified to offer Novabel that they can continue to use it in accordance with the pack insert if they wish. However, if they should decide not to use any remaining product that they have in their possession Merz will fully refund all unused returned products.
The company asks for continued diligence in the monitoring of patients previously injected with Novabel, whilst its R&D team identifies an effective treatment or management protocol for these rare events.
Merz remains very optimistic and excited about the unique benefits Novabel offers to practitioners and patients, and note that their aim is to confidently promote Novabel when they can provide everyone with reassurance that there are effective treatment options for those rare potential adverse reactions.
Hey, wait!
Before you go.....
Let's stay in touch, pop your details here and we'll send our editor's hand-picked updates on your fave subjects.