 Product Summary
Product Summary
Juvéderm® ULTRA is an advanced, cohesive, 3D hyaluronic acid matrix dermal filler with local anaesthetic, manufactured by Allergan; the makers of Botox® / Vistabel® and Juvéderm® VOLUMA.
Juvéderm® ULTRA was launched in the UK in February 2008.
Generic name
Cross-linked hyaluronic acid plus lidocaine, (also called lignocaine in the UK).
What does it contain?
Juvéderm® ULTRA contains non-animal hyaluronic acid with the addition of 0.3% lidocaine, local anaesthetic to provide more comfort both during and after injection.
However, in the case of lip augmentation it may still be recommended that you have a topical anaesthetic or dental nerve block in order to ensure a pain free treatment of this sensitive area.
According to data from the manufacturer concerning the preliminary results from a study on 60 women, which compared the injection comfort of Juvéderm® ULTRA 3 and Surgiderm 30 XP, 81% who received Juvéderm® ULTRA felt only slight or no discomfort at all.(1)
How is it made?
By bacterial fermentation from streptococci bacteria.
Is a skin test required before treatment?
No allergy test needed.
Is it temporary or permanent?
Hyaluronic acid is completely broken down within the skin over a period of months, eventually leaving no trace of the filler.
Licenced status
Medical device.
Should be used by
Medically trained practitioners – ie Surgeons, Doctors, Dentists & Nurses.
Product range 
There are four different formulations for Juvéderm® ULTRA available which include:
Juvéderm® ULTRA 2
(2 x 0.55ml syringes, 30G needle)
A highly cross-linked formulation for the subtle correction of medium facial lines and skin depressions. Also enhances lip contour.
Juvéderm® ULTRA 3
(2 x 0.55ml syringes, 27G needle)
A highly cross-linked formulation for more versatility in contouring and volumising medium to deep facial lines and skin depressions, such as nasolabial folds. Also enhances lip contour and volume.
Juvéderm® ULTRA 4
(2 x 0.8ml syringes, 27G needle)
A highly cross-linked robust formulation for volumising and correction of deeper folds and wrinkles, including enhancing volume in the cheeks and chin. This formulation should not be injected into the glabella (frown lines) or into the areas under the eyes. 
Juvéderm® ULTRA Smile
(2 x 0.55ml syringes, 27G needle)
The newest member of the Juvéderm® ULTRA family was launched in January 2010, based on the Juvéderm® ULTRA 3 product it is specifically targeted for use in the lips.
Not to be used in
Individuals with a known hypersensitivity to hyaluronic acid or lidocaine.
Patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies.
Juvéderm® ULTRA should not be administered directly after laser treatment, deep chemical peeling, dermabrasion or in the case of superficial chemical peeling if there is a severe inflammatory reaction.
Duration of effect
According to the manufacturer Juvéderm® ULTRA has enhanced stability and resistance to degradation by heat and the body’s metabolism, providing a duration of the product up to 12 months.
Reported side effects
Transient erythema (redness), swelling, pain, itching, discolouration or tenderness at the implant site.
Typically resolution is spontaneous, within one or two days after injection into the skin, and within a week after injection into the lips.
Costs
This depends on the area treated and how much is required, and the practitioner doing the treatment, but price ranges are in the region of £275+ per pack of Juvéderm® ULTRA.
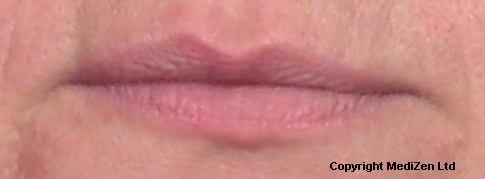
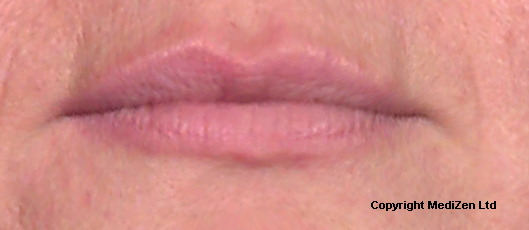
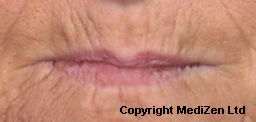
Before and After Photos of Juvéderm® ULTRA
 |
 |
 |
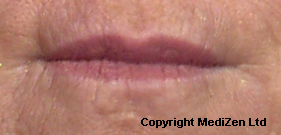 |
| Left Images show Lips pre-treatment with Juvederm | Improvement in lip lines, volume, shape and profile following treatment with Juvederm |
Images above provided courtesy of MediZen Ltd
Before and after photographs are real patients, your results may differ.
Clinical Trials
(1) Comparison of Injection Comfort and Ease with Juvéderm ULTRA 3 and Surgiderm 30 XP. Phillip Levy, Hervé Raspaldo, Koenrad De Boulle. Accepted as a poster for IMCAS Jan 9-12, 2008, Paris.
Do you have a question? Ask one of our experts NOW