In 2016, the EMERVEL range, also from Galderma was integrated into their Restylane range to create one large range of hyaluronic acid fillers. This meant that EMERVEL ceased as a brand name and all products were renamed. Please refer to the Restylane page for further information.

Product Summary
EMERVEL® is a range of five cross-linked hyaluronic acid based dermal fillers from Q-Med, a Division of Galderma, the UK distributor of the Botulinum Toxin brand Azzalure and the dermal filler brand Restylane. It was introduced to the UK and Europe in 2011.
Emervel dermal fillers are indicated for the treatment of facial lines, for facial contouring and the correction of volume loss, as well as lip enhancement.
They are also available with the addition of local anaesthetic lidocaine for added comfort during injection.
Generic name
Non-animal cross-linked hyaluronic acid.
What does it contain?
The main component of Emervel is hyaluronic acid. This is a naturally occurring substance found in all living organisms. The hyaluronic acid is mixed with a cross-linker called Butanediol Diglycidyl Ether (BDDE) to form a gel, which is then purified through dialysis to remove trace amounts of BDDE left over from the process.
How is it made?
The hyaluronic acid in Emervel is produced by a biotechnological process from bacterial fermentation without using any animal products.
Emervel benefits from what the manufacturers call its Optimal Balance Technology™.
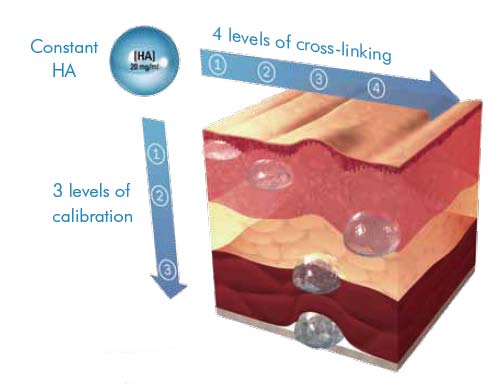
Their production method is designed to alter the characteristics of the hyaluronic acid gel via a combination of factors, including the levels of cross-linking (a process which is needed to ensure the hyaluronic acid is long lasting), the particle sizes, which are varied to product the differing products within the range which aim to have a maximum effect in the different layers of the skin into which they are injected.
Emervel has 4 levels of cross-linking, resulting in 4 levels of gel firmness across the range. A lower level of cross-linking will result in a softer gel suitable for superficial injection and a higher level of cross-linking results in a firmer gel more resistant to degradation for deeper injection. Within the range, Touch, Classic and Lips share the same smaller particle size with Deep and Volume using increasingly larger particle sizes for deeper skin application. Particle size or calibration is achieved during the manufacturing process after cross-linking, by sieving the gels through different sizes of mesh.
The actual concentration of the hyaluronic acid is the same throughout the range at 20mg/ml.
Some hyaluronic acid based fillers require what’s called free or non-cross-linked hyaluronic acid within the product in order to modify the gel viscosity (thickness) and aid in the extrusion or pushing out of the product through the syringe and needle. However, free hyaluronic acid is simply a liquid and has no filling properties because it is metabolised by the body quickly upon injection. Also by having a good affinity for water molecules, much like a sponge, the addition of it can result in increased swelling in the area upon injection. The manufacturers note that the viscoelastic properties of Emervel are produced by modifying the cross-linking and particle sizes, as detailed above and not the concentration of hyaluronic acid and thus no free hyaluronic acid is incorporated which they claim makes swelling (oedema) on injection minimal.
Is a skin test required before treatment?
No.
Is it temporary or permanent?
Hyaluronic acid is completely broken down within the skin over a period of months, eventually leaving no trace of the filler.
Licenced status
Medical device
Should be used by
Trained members of the medical profession only
Product range
There are 5 different formulations for Emervel available which include:
Emervel Touch

Emervel Touch is a soft and subtle formulation for the treatment of superficial wrinkles, such as peri-oral lines (around the mouth) and peri-orbital lines (around the eyes, crow’s feet). It is injected into the superficial dermis and is available in a prefilled 1 x 1ml syringe and comes complete with 2 x 30G ½ `Ultra Thin Wall` needles. It is not available with the addition of lidocaine.
Emervel Classic

Emervel Classic provides a medium lifting effect and is ideal for moderate lines and wrinkles, such as the naso-labial folds (nose-to-mouth lines). The addition of 0.3% lidocaine ensures comfort during treatment. It is injected into the dermis and is available in a prefilled 1 x 1 ml syringe and comes complete with 2 x 30G ½ `Ultra Thin Wall` needles.
Emervel Lips

Emervel Lips is specifically formulated to enhance, define or augment the lip body and border. The addition of 0.3% lidocaine ensures comfort during treatment. It is injected into the body of the lip and the border and is available in a prefilled 1 x 1 ml syringe and comes complete with 2 x 30G ½ `Ultra Thin Wall` needles.
Emervel Deep

Emervel Deep has a high volume lifting effect and integrates with the skin matrix to smooth away deep wrinkles or folds such as naso-labial and marionette lines (mouth-to-chin lines). The addition of 0.3% lidocaine ensures comfort during treatment. It is injected into the deep dermis or upper subcutaneous layer and is available in a prefilled 1 x 1 ml syringe and comes complete with 2 x 27G ½ `Ultra Thin Wall` needles.
Emervel Volume

Emervel Volume also has a very high volume lifting effect and integrates with the skin matrix to address lost facial volume associated with ageing. It is suited to patients wishing to enhance or recontour their facial features such as the cheekbones, cheeks, chin, or the whole facial oval. The addition of 0.3% lidocaine ensures comfort during treatment. It is injected into the subcutaneous layer and is available in a prefilled 1 x 2 ml syringe and comes complete with 2 x 23G 1 `Ultra Thin Wall` needles.
Not to be used in
Treatment is not recommended for women who are pregnant or breastfeeding.
Emervel dermal fillers are contraindicated in patients presenting with severe allergies, particularly those with a known allergy or sensitivity to hyaluronic acid, as well as autoimmune disorder, porphyria and hypersensitivity to lidocaine or amide anaesthetics (found in lidocaine formulations).
Treatment with dermal fillers should also be avoided in areas of skin with acute or chronic lesions, or where other fillers, particularly permanent ones have already been injected.
Duration of effect
The Optimal Balance Technology™, employed in the manufacture of Emervel, provides a product which lasts for 6 - 9 months. In clinical trials, some effects have also been seen at 12 months.
Reported side effects
Swelling, skin redness (erythema), mild pain, tenderness and itching are among the common side effects after injection with dermal fillers such as Emervel. These usually disappear within a few days after treatment.
Costs
This depends on the area(s) treated, the specific product within the range used and how much is required, as well as the practitioner doing the treatment, but price ranges are in the region of £200 - £350 per syringe, with additional syringes used in the same treatment session often at a discount.
Clinical Results
Emervel has been tested in two multicentre, randomised, evaluator-blinded, European clinical trials. Subjects were treated with two different fillers (Emervel vs competitor filler), one on each side of their face.
The first study compared Emervel® Classic with Restylane® on 52 people in the treatment of moderate facial lines. Emervel Classic was found to be as effective as Restylane at 12 and 24 weeks. It was well tolerated, the majority of people reported none or only mild injection site reactions and it was associated with significantly less erythema or redness, less oedema or swelling and less pain or tenderness than Restylane (based on patient diary worst scores during the first 3 weeks after baseline injection).
The second study compared Emervel® Deep with Perlane® on 68 people in the treatment of severe facial lines. Emervel Deep was found to offer significantly better efficacy than Perlane at all visits from week 12 to week 48. The majority of reactions with Emervel Deep were mild and resolved spontaneously without additional treatment.
Clinical Data
The Emervel French survey: a prospective real-practice descriptive study of 1,822 patients treated for facial rejuvenation with a new hyaluronic acid filler.
Farhi D, Trevidic P, Kestemont P, Boineau D, Cartier H, Bodokh I, Brun P, Ascher B, Savary J; Emervel French Survey Group. Dermatology Clinic, 75013 Paris, France.
J Drugs Dermatol. 2013 May;12(5):e88-93.
Full-face rejuvenation using a range of hyaluronic acid fillers: efficacy, safety, and patient satisfaction over 6 months.
Rzany B, Cartier H, Kestemont P, Trevidic P, Sattler G, Kerrouche N, Dhuin JC, Ma YM.
Dermatol Surg. 2012 Jul;38(7 Pt 2):1153-61.
Emervel®: full-face rejuvenation with a range of customized hyaluronic acid fillers.
Rzany B.
J Drugs Dermatol. 2012 Jan;11(1 Suppl):s4.
A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations.
Segura S, Anthonioz L, Fuchez F, Herbage B. Galderma R&D, Sophia Antipolis, France.
J Drugs Dermatol. 2012 Jan;11(1 Suppl):s5-8.
Sustained efficacy and high patient satisfaction after cheek enhancement with a new hyaluronic acid dermal filler.
Kestemont P, Cartier H, Trevidic P, Rzany B, Sattler G, Kerrouche N, Dhuin JC. CHU Pasteur, Head and Neck Surgery, Nice, France.
J Drugs Dermatol. 2012 Jan;11(1 Suppl):s9-16.
Perioral rejuvenation with a range of customized hyaluronic acid fillers: efficacy and safety over six months with a specific focus on the lips.
Cartier H, Trevidic P, Rzany B, Sattler G, Kestemont P, Kerrouche N, Dhuin JC. Centre Medical Saint Jean, Arras, France.
J Drugs Dermatol. 2012 Jan;11(1 Suppl):s17-26.
Correction of tear troughs and periorbital lines with a range of customized hyaluronic acid fillers.
Rzany B, Cartier H, Kestermont P, Trevidic P, Sattler G, Kerrouche N, Dhuin JC. Division of Evidence Based Medicine, Charité-Universitätsmedizin, Berlin, Germany.
J Drugs Dermatol. 2012 Jan;11(1 Suppl):s27-34.
Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study.
Rzany B, Bayerl C, Bodokh I, Boineau D, Dirschka T, Queille-Roussel C, Sebastian M, Sommer B, Poncet M, Guennoun M, Podda M. dEBM, Charité-Universitätsmedizin, Berlin, Germany.
J Cosmet Laser Ther. 2011 Jun;13(3):107-12.
Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines - 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study.
Ascher B, Bayerl C, Brun P, Kestemont P, Rzany B, Poncet M, Guennoun M, Podda M. Clinique de Chirurgie Esthétique Iéna, 11 Rue Fresnel, Paris, France.
J Cosmet Dermatol. 2011 Jun;10(2):94-8.